
Zero Liquid Discharge (ZLD)
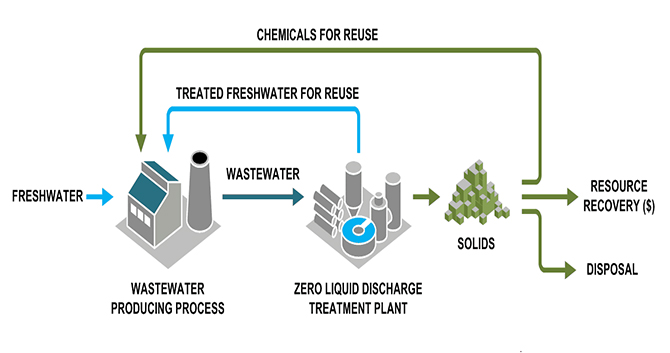
Zero-liquid discharge (ZLD) is a water treatment process in which all wastewater is purified and recycled; therefore, leaving zero discharge at the end of the treatment cycle. It ensures that there will be no discharge of industrial wastewater into the environment with zero liquid waste. This ultimate treatment method has zero or nearly zero negative impaction the environment.
There are especially 4 additives that might be very crucial for a structured ZLD process:
- Pre-Treatment (Biological & physicochemical)
- Biological Treatment
- Polishing Treatment - (RO) Reverse Osmosis (Membrane methods) - Evaporator and crystallizer (thermal processes)
Application of ZLD in Industries
ZLD technology includes pre-treatment and evaporation of the industrial effluent until the dissolved solids precipitate as crystals. These crystals are removed and dewatered with a filter press or a centrifuge. Zero liquid discharge is an advanced wastewater treatment method that includes Polishing/ Tertiary treatment such as:
- Ultrafiltration
- Reverse osmosis (RO)
- Evaporation/crystallization (MEE/MVR+ATFD+Spray Dryer)
- Electrodialysis (ED/EDR)
- Forward osmosis (FO)
- Membrane distillation (MD)
- Fractional electrode ionization
- ion exchange
The significant industrial sectors like Pharma (API and Formulation), Pesticide (API and Formulation), Speciality chemicals, Petrochemicals, Sugar, Distilleries, Tanneries, Pulp & Paper, Textile, Dyeing, and Dairy would need special emphasis for enforcement of ZLD. ZLD is applicable to industries having high BOD and COD load, colour bearing effluents, having metals, pesticides and other toxic / hazardous constituents.
Multi Effect Evaporator (MEE)
The Multiple Effect Evaporator (MEE) is an apparatus for efficiently using the heat from steam to evaporate water. In a multiple-effect evaporator, water is boiled in a sequence of vessels, each held at a lower pressure than the last.
Evaporators are kind of heat transfer equipments where the transfer mechanism is controlled by natural convection or forced convection. A solution containing a desired product is feed into the evaporator and it is heated by a heat source like steam. Because of the applied heat, the water in the solution is converted into vapour and is condensed while the concentrated solution is either removed or feed into a second evaporator for further concentration. If a single evaporator is used for the concentration of any solution, it is called a single effect evaporator system and if more than one evaporator is used for the concentration of any solution, it is called a multiple effect evaporator system.


Mechanical Vapor Recompression (MVR)
Mechanical Vapor Recompression (MVR) is a proven energy-saving evaporative concentration technology, which reduces evaporation energy use by 90% or more. MVR uses energy recovered from the condensate to create a pure liquid distillate and a concentrated product/waste. The energy normally lost in the compression is recovered, leading to a highly-efficient evaporation process.
Mechanical Vapor Recompression (MVR) evaporation process involves forced circulation flash evaporator utilizing mechanical vapor compression. Minimal scaling, maximum uptime, and low operating costs are the take away points of MVR design.
Water Vapor evaporated from effluent is recompressed with a simple, low speed centrifugal fan. The fan increases the saturation temperature of the vapor and hence the vapor can be used for heating in the same effect. The recompressed vapor condenses and releases its latent heat through heat transfer surface for further evaporation of effluent.
MVR systems are operated under vacuum and typically boil water at 60 C. Since the evaporation takes place at lower temperature there is a much lower chance of organics carryover in the system.
Industrial RO system
The most important factor in treating industrial wastewater with Reverse Osmosis is the pretreatment that protects the membrane against organic fouling, mineral scaling and chemical degradation. Before reverse osmosis should be considered, a complete cation/anion balance is required and potential foulants must be identified. High BOD and COD levels can also contribute to membrane fouling. A wide range of pretreatment technologies is available.
Reverse osmosis (RO) and Nanofiltration (NF) membranes are commonly used as a filtration method to remove many types of dissolved solids (large molecules and ions) from solutions by applying pressure to the solution when it is on one side of a selective membrane. The result is that the solute is retained on the pressurized side of the membrane and the pure solvent is allowed to pass to the other side. Reverse Osmosis is the reversal of this natural phenomenon, by the application of external pressure on the solution that contains the higher concentration of dissolved ions, thus forcing water through the semi-permeable membrane in the opposite direction, leaving behind the dissolved ions and the suspended solids.

In the reverse osmosis process, the water that passes through the membrane is commonly referred to as permeate, or product water. The water that remains behind the membrane along with the dissolved and suspended solids is referred to as the reject, brine, or concentrate water. Most applications looking for high salt rejection resulting in lower higher quality effluent typically utilize RO membranes. In some applications where high salt rejection is not required, Nanofiltration membranes (often referred to as softening membranes) can be used to selectively remove certain salts from the feed stream reducing the total dissolved solids to acceptable levels at lower operating pressures than RO membranes
Spray Dryer
Spray drying is the transformation of feed from a fluid state into a dried particulate form by spraying the feed into a hot drying medium. A spray dryer operates on convection mode. The principle of working is moisture removal by application of heat to the feed product and controlling the humidity of the drying medium. Here, the uniqueness is that the evaporation of moisture is promoted by spraying the feed into a heated atmosphere, resulting in improved drying rate. The mechanism can be better understood, when the spray drying process is divided into its constituent unit operations. A liquid feed entering the spray dryer undergoes a series of transformations before it becomes powder.

Ion Exchange Application in Wastewater Treatment
Ion Exchange can be used in wastewater treatment plants to swap one ion for another. Regardless of which type of exchangers, the resins can very sensitive to fouling caused by presence of organic matter in the untreated water. Treatment of the effluent by a column of anion exchange resin might be expected to achieve reductions in the levels of nitrate and phosphate by ion exchange, dissolved organics by adsorption and suspended solids by filtration.

Nutrient Removal
The reduction in levels of Nitrate and phosphate nutrients would be of benefit in many effluents. Regulated phosphorus & nitrogen discharges and the cost of fertilizer provide economic drivers for nutrient removal and recovery from wastewater
Organic matter
The uptake of organic matter by the resins may be regarded as a beneficial side effect of ion exchange treatment. Adsorption and/or ion exchange of dissolved constituents are predominant mechanisms for organic removal.
Colour
The role of ion exchange in dye effluents treatment is to reduce the magnitude of hazardous load by converting them into a form in which they can be reused. Many resins exhibits promising adsorbent properties for the textile wastewater treatment.
Phenol
The removal of phenol from Industrial effluent using ion exchange resin is commercialized now. Uptake of phenol onto ion exchange resin occurs by both adsorption and ion exchange at alkaline pH (in Clform). The ion exchange kinetic of Cl form is much faster than the OH form.
Ammonia
Ion exchange offers a number of advantages in ammonia removal including the ability to handle shock loadings and to operate over a wider range of temperatures. Natural zeolites such as clinoptilolite shows better results in presence of organic molecules.

